We will discuss the following aspects. Please scroll down and start reading.
- Introduction
- Oxygen Saturation
- Physical properties used in pulse oximetry
- Calibration
- Pulse oximeters measure pulsatile arterial blood
- The signal is tiny
- Plethysmographic trace (pleth)
- Light source
- Coping with ambient light
- Problems associated with using pulse oximeters.
Introduction
Pulse oximeters measure the percentage of haemoglobin in blood that is carrying oxygen (oxygen saturation).
If you work in healthcare (or have been a patient), you are likely to have come across pulse oximeters. You can find them in areas such as operating rooms, recovery rooms, critical care units, wards, and ambulances.
Pulse oximeters are in common use because they are:
- Non-invasive
- Cheap to buy and use
- Can be very compact
- Detects hypoxaemia earlier than you can visually detect cyanosis.
Oxygen Saturation
Pulse oximeters measure oxygen saturation. Before we discuss how pulse oximeters work, we must first understand what oxygen saturation is. If you already know what oxygen saturation is, skip this section by clicking here. Otherwise, here is a very brief description of it.
We all know that we need oxygen for life. Oxygen in the air must be delivered to the various cells in one’s body to survive.
Oxygen enters the lungs and is passed on into the blood, which carries oxygen to the various organs in our body. The primary way oxygen is carried in our blood is through haemoglobin. You can imagine haemoglobin molecules( Hb) as “cars” and the “roads” as our blood vessels. The oxygen molecules enter these cars and travel around the body until they reach their destination.

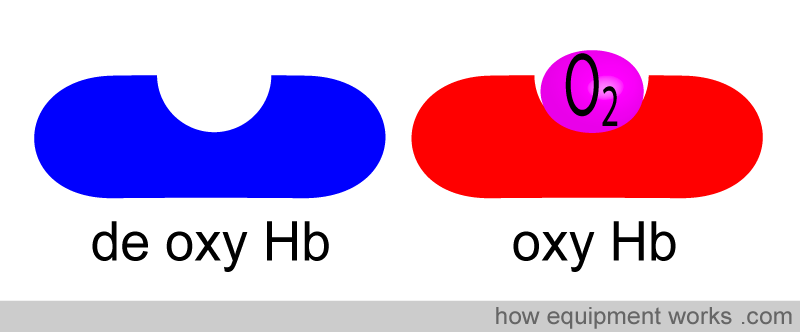
Let us consider these “haemoglobin cars”.
The haemoglobin without oxygen we will call de-oxygenated haemoglobin (deoxy Hb). The haemoglobin with oxygen, we will call oxygenated haemoglobin (oxy Hb).
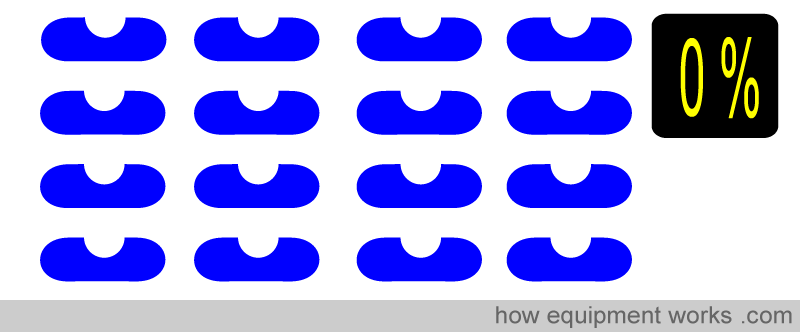
Oxygen saturation simply refers to the percentage of the available haemoglobin that carries oxygen. Take the situations below. There are 16 haemoglobin units, and none of the 16 have oxygen. The oxygen saturation is therefore 0 %.
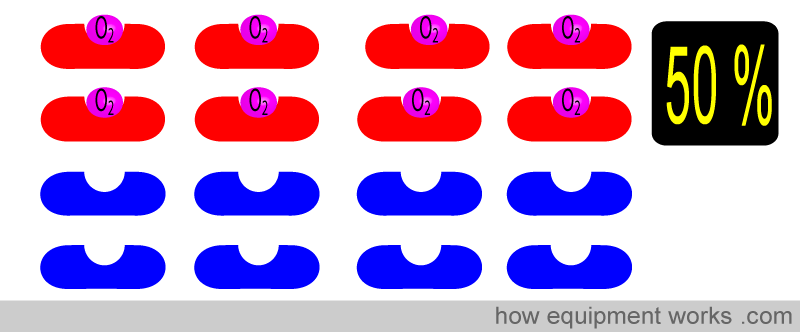
Here, 8 of the 16 Hb have oxygen. The oxygen saturation is therefore 50 %.
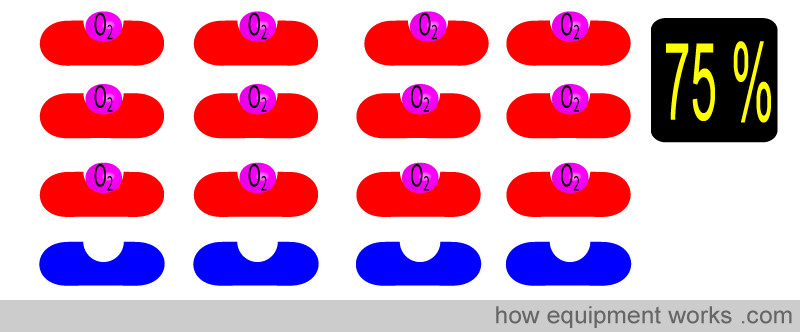
Similarly for 75 % …
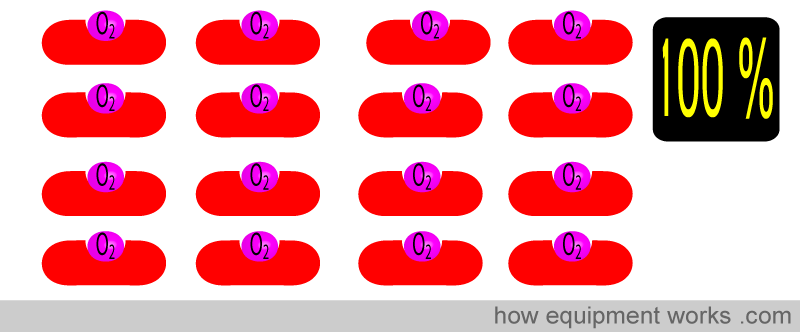
And of course, when all the Hb have oxygen, the saturation is 100 %
So, in summary, oxygen saturation tells you the percentage of the total haemoglobin that is carrying oxygen.
Physical properties used in pulse oximetry
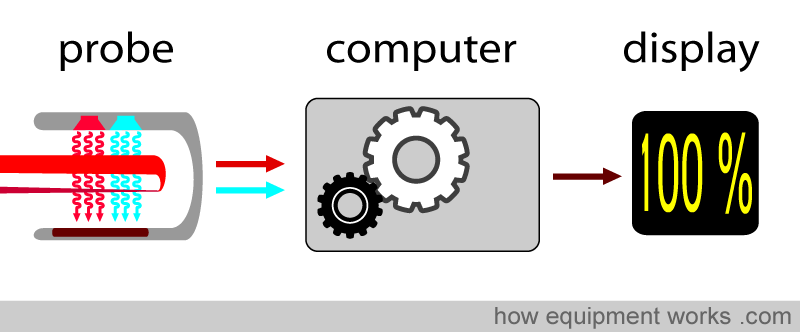
Pulse oximetry uses light to work out oxygen saturation. Light is emitted from light sources, which goes across the pulse oximeter probe and reaches the light detector.
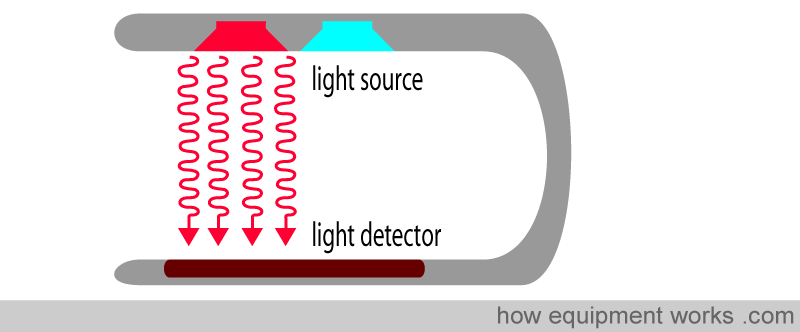
If a finger is placed between the light source and the light detector, the light must now pass through the finger to reach the detector. The finger will absorb part of the light, and the part not absorbed reaches the light detector.
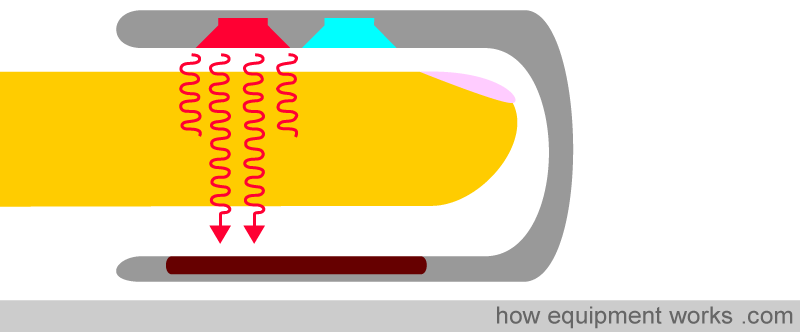
The amount of light absorbed by the finger depends on several physical properties, which the pulse oximeter uses to calculate oxygen saturation.
The amount of light absorbed depends on the following:
1. Concentration of the light-absorbing substance.
2. Length of the light path in the absorbing substance
3. Oxyhemoglobin and deoxyhemoglobin absorb red and infrared light differently
Don’t worry! All the above will be explained in the next sections:
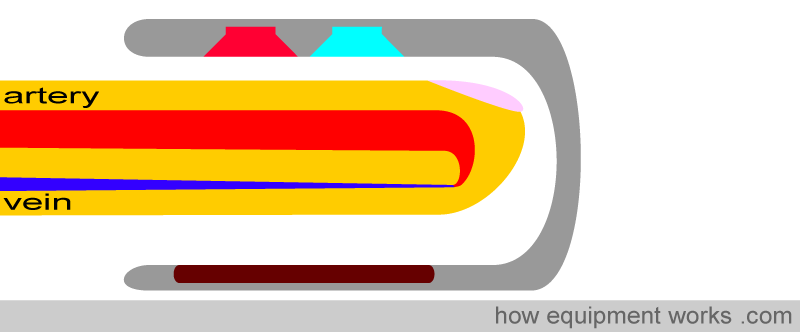
The physical properties that a pulse oximeter employs will be explained using the probe shown below. A finger is shown inserted into the probe. Above the finger are the light sources that emit light. In the finger is an artery which carries the blood the pulse oximeter is interested in, and a vein through which the blood leaves the finger. Below the finger is the light detector.
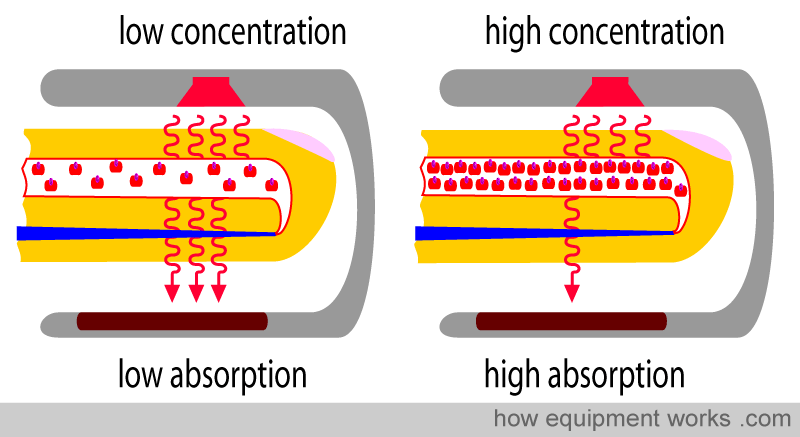
Physical property No.1: The amount of light absorbed is proportional to the concentration of the light-absorbing substance
Haemoglobin (Hb) absorbs light. The amount of light absorbed is proportional to the concentration of Hb in the blood vessel. In the diagram below, the blood vessels in both fingers have the same diameter. However, one blood vessel has a low Hb concentration (i.e., a low number of Hb in each unit volume of blood), and the other blood vessel has a high Hb concentration (i.e., a high number of Hb in each unit volume of blood). Each single Hb absorbs some of the light, so the more the Hb per unit area, the more light is absorbed. This property is described in a law in physics called “Beer’s Law”.
Beer’s Law: The amount of light absorbed is proportional to the concentration of the light-absorbing substance
By measuring the amount of light reaching the light detector, the pulse oximeter determines how much light has been absorbed. The more the Hb in the finger, the more light is absorbed.
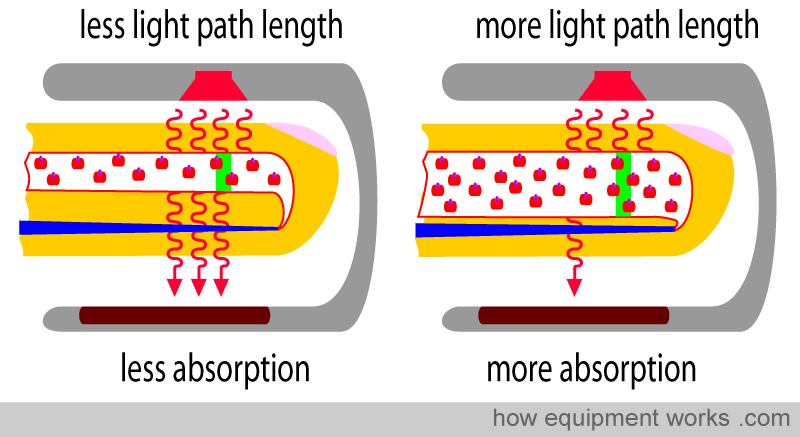
Physical property No.2: The amount of light absorbed is proportional to the length of the light path.
Look at the two fingers shown below. Both arteries have the same concentration (same Hb per unit area, indicated by the blue square). However, the artery on the right is wider than the one on the left.
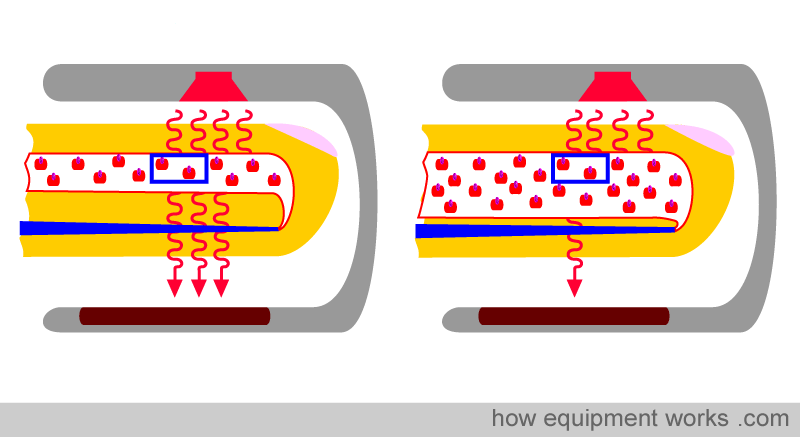
The light emitted from the source has to travel through the artery. The light travels a shorter path in the narrow artery and a longer path in the wider artery (paths are shown as green lines below). Although the concentration of Hb is the same in both arteries, the light encounters more Hb in the wider artery, as it travels a longer path. Therefore, the longer the path the light has to travel, the more light is absorbed. This property is described in a law in physics called “Lambert’s Law”.
Lambert’s Law: The amount of light absorbed is proportional to the length of the path that the light has to travel in the absorbing substance.
Physical property No.3: oxyhemoglobin absorbs more infrared light than red light & deoxyhemoglobin absorbs more red light than infrared light (this is explained below !)
We have seen how concentration and light path affect the absorbance of light. In addition to these, the pulse oximeter utilises another essential property to calculate oxygen saturation. That is, oxyhemoglobin and deoxyhemoglobin absorb light of different wavelengths in a specific way.
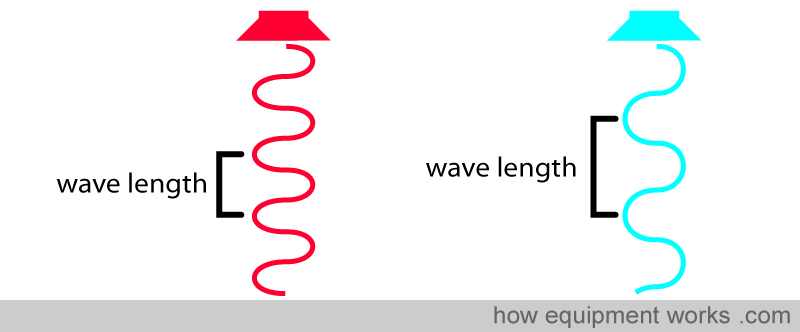
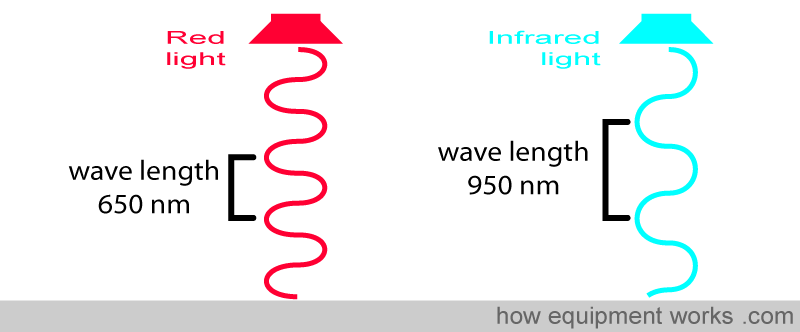
Before we proceed, let’s recall what a wavelength is. All light is composed of waves. The distance between the “tips” of the waves is equal to the wavelength.
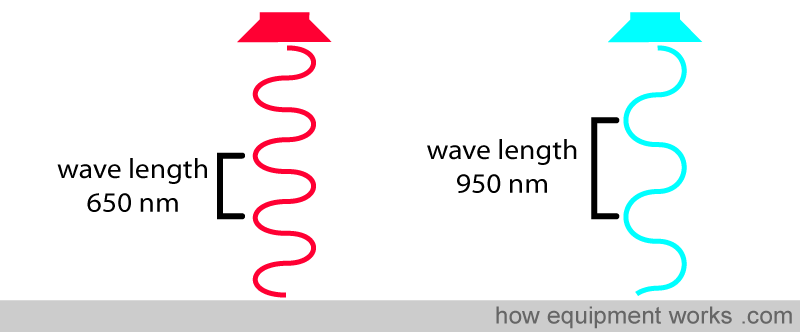
Light wavelengths are very short, and the unit of measurement is in nanometers (nm) (1 meter = 1,000,000,000 nanometers). For example, the wave on the left has a wavelength of 650 nm, and the wave on the right has a longer wavelength of 950 nm.
Different “colours” of light have different wavelengths.
The pulse oximeter uses the property that oxyhemoglobin and deoxyhemoglobin absorb light of different wavelengths in a specific way. This property can be demonstrated in a laboratory, as described below. We can first demonstrate how oxyhemoglobin absorbs light of different wavelengths in a particular way. We use a special light source from which we can adjust the wavelength of the light it emits. This light source sequentially passes light of different wavelengths through a sample of oxy Hb. The detector notes how much light, at each wavelength, has been absorbed.
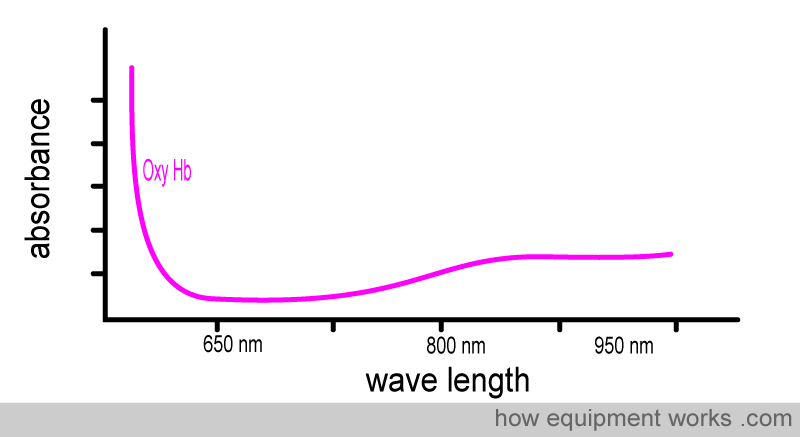
A graph for the absorbance of oxyhemoglobin at different wavelengths will look like this. It shows that oxy Hb doesn’t absorb the same amount of light at different wavelengths.
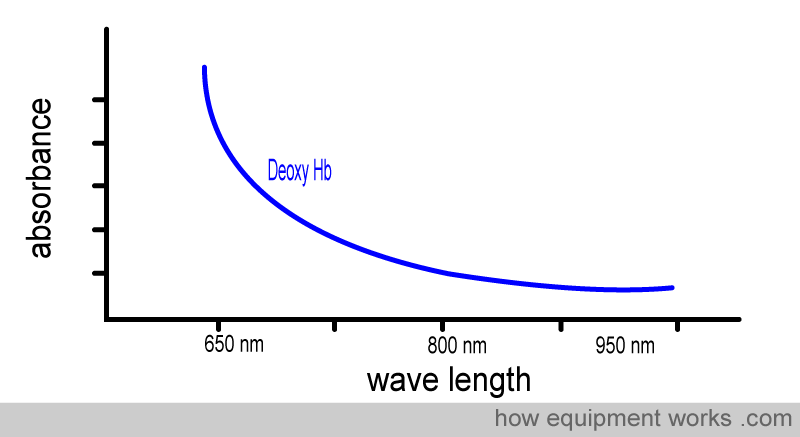
We can repeat the same demonstration using deoxy Hb.
Again, notice how, like oxy Hb, Deoxy Hb absorbs different amounts of light at different wavelengths.
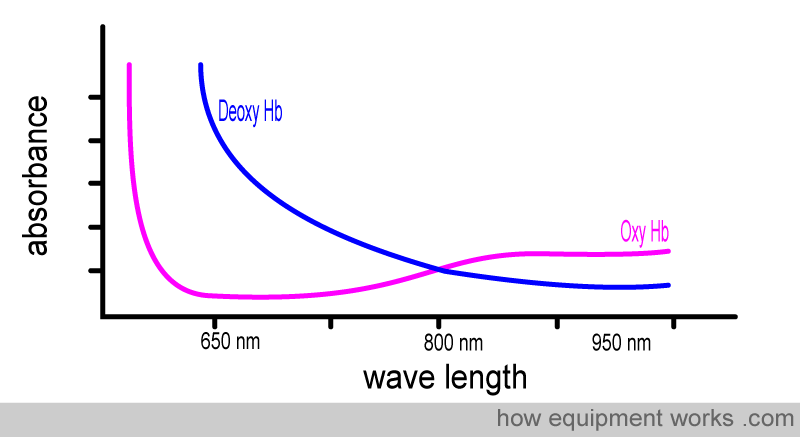
Now, let’s examine the absorbance graphs of oxy Hb and deoxy Hb together, allowing you to compare them. Note how each of them absorbs light of different wavelengths very differently.
I am the author of this website, which I hope you find useful. Let me tell you about another website I run that you may like. In addition to my interest in teaching about medical equipment, I am very interested in psychology related to happiness! I have created a website that explains a concept called “Happy Thinking”, which is about thinking in ways that promote happiness in oneself. The website is free, and you are welcome to visit it at the link below.

The pulse oximeter uses two lights to analyse haemoglobin.
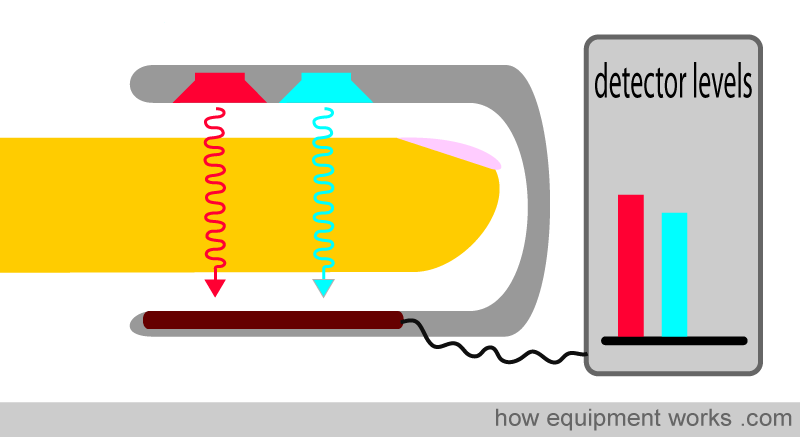
One is a red light, which has a wavelength of approximately 650 nm. The other is infrared light, which has a wavelength of 950 nm. Throughout our description, we will show the infrared light in light blue. In reality, infrared light is invisible to the human eye.
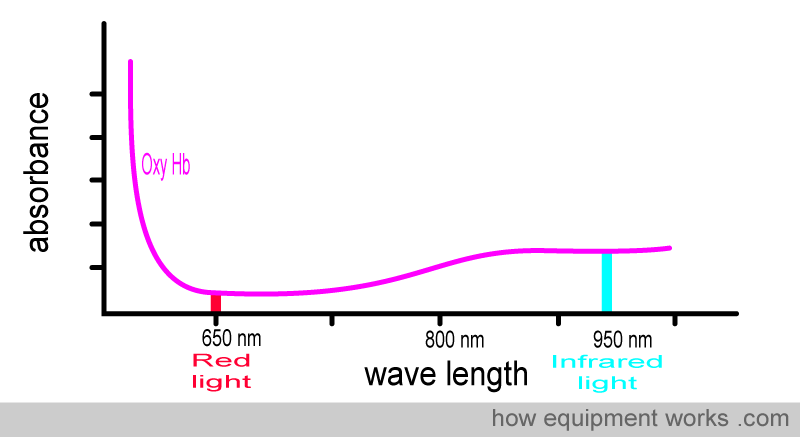
Now look at the oxy Hb absorbance graph again, but this time pay attention to the wavelengths of light used in pulse oximeters. You will see that oxy Hb absorbs more infrared light than red light.
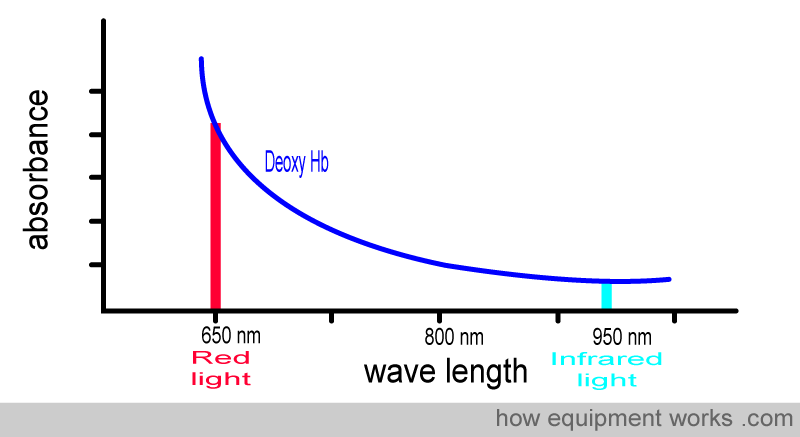
Below is the graph showing the absorbance of deoxy Hb. It is seen from the graph that deoxy Hb absorbs more Red light than Infrared light.
To facilitate the comparison of the absorbance of oxy-Hb and deoxy-Hb, a composite graph is provided below, showing the absorbance of both. You will see that :
Oxy Hb absorbs more infrared light than red light
Deoxy Hb absorbs more red light than infrared light
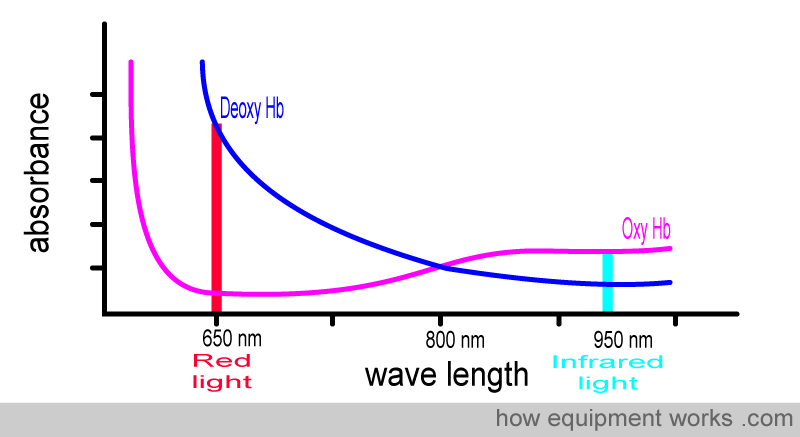
You may find the memory aid below helpful in remembering the wavelengths absorbed by oxy Hb and deoxy Hb.
The pulse oximeter determines oxygen saturation by measuring the amount of red light and infrared light absorbed by the blood. Depending on the amounts of oxy Hb and deoxy Hb present, the ratio of the amount of red light absorbed compared to the amount of infrared light absorbed changes.
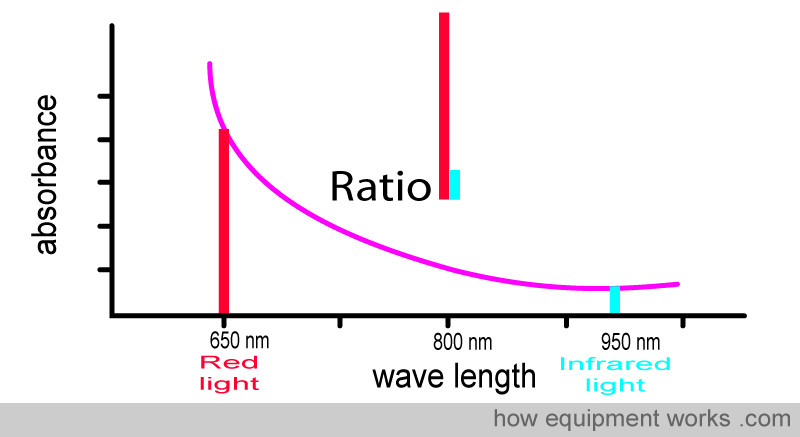
Using this ratio, the pulse oximeter can then work out the oxygen saturation.
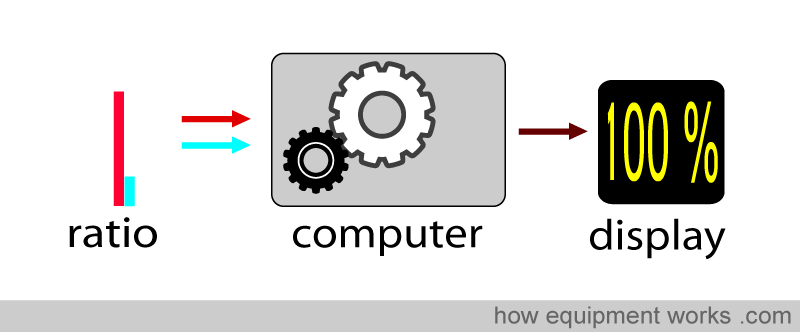
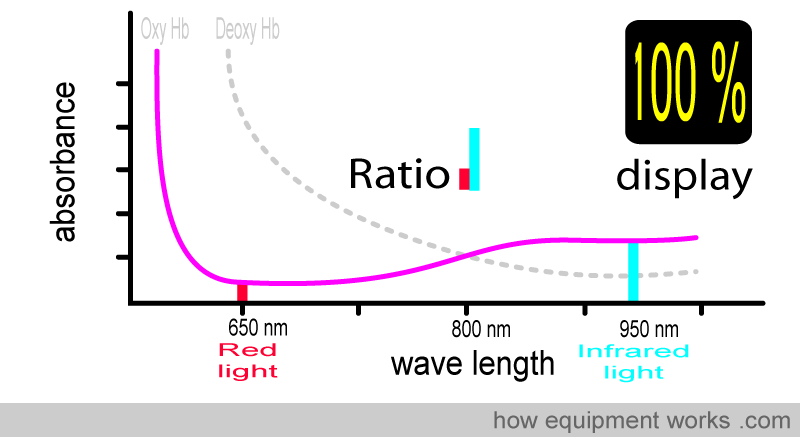
For example, at 100% saturation, the absorbance ratio (i.e., comparing how much red light and infrared light are absorbed) will be the same as that seen with the oxy Hb absorbance curve that we saw earlier.
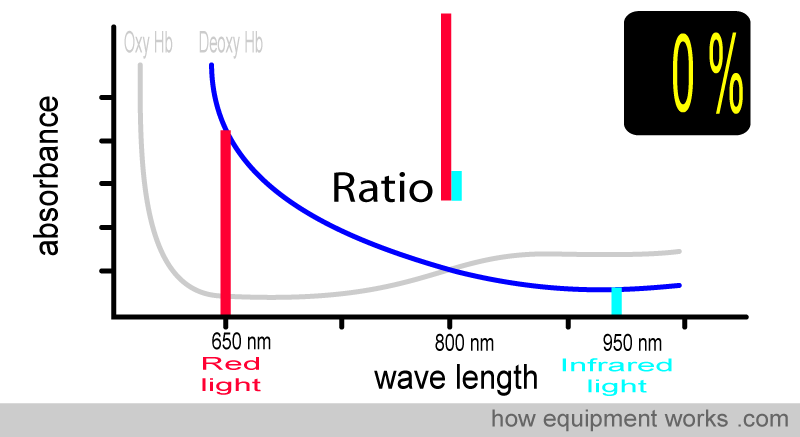
At 0 % saturation, there is only deoxy Hb. The absorbance ratio (i.e., comparing how much red light and infrared light are absorbed) will therefore be the same as that seen with the deoxy Hb absorbance curve that we saw earlier.
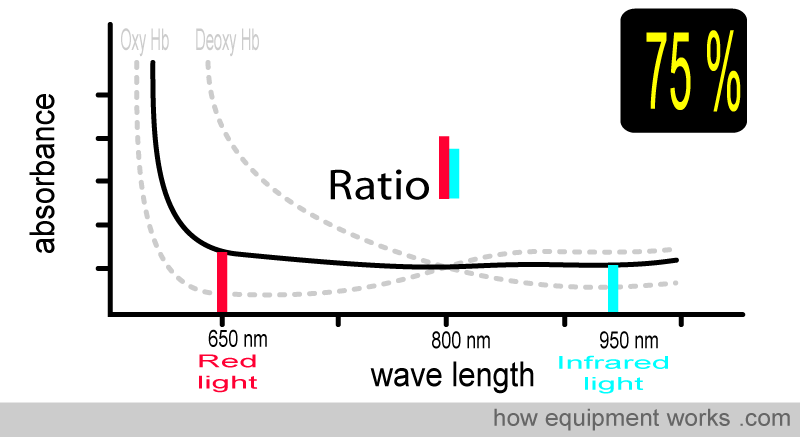
Now, consider the case where the patient has an oxygen saturation of 75%. The blood contains both oxy Hb and deoxy Hb. The absorbance pattern is now positioned somewhere between the oxy Hb curve and the deoxy Hb curve (both shown in grey). The ratio of absorbed red light and infrared light is different, and using this information, the pulse oximeter calculates the oxygen saturation as 75 %.
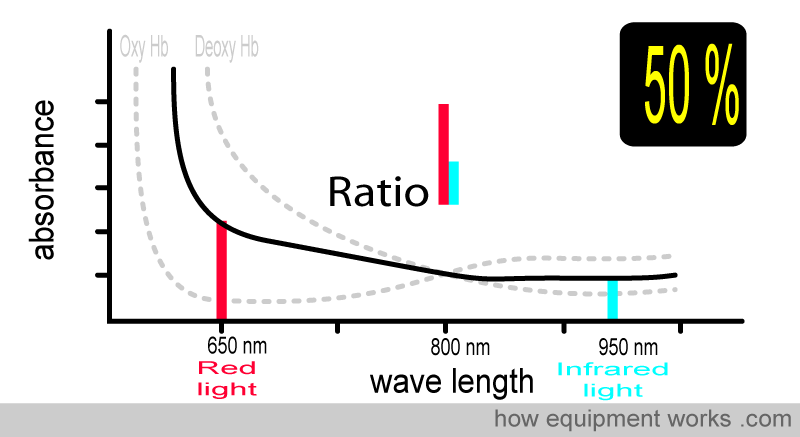
At 50% oxygen saturation, the absorbance pattern differs from when the saturation was 75%. The ratio of red light and infrared light absorbed is also therefore different, and the pulse oximeter uses this to calculate the saturation as 50%.
At 25 % saturation, you and your patient are in deep trouble. Again, the ratio is different.
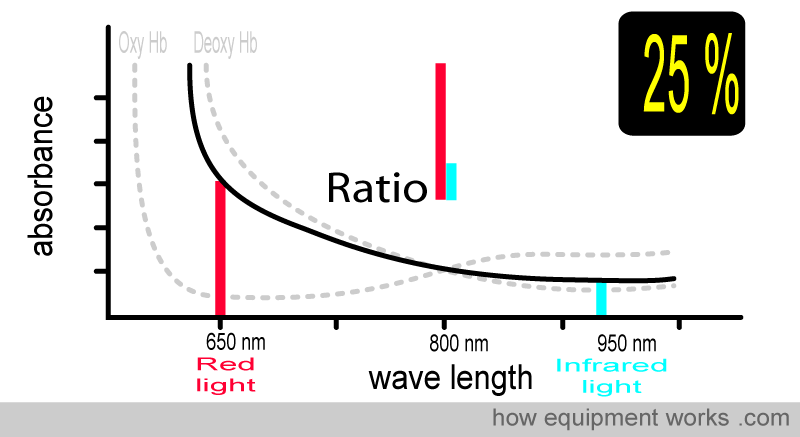
The animation below shows what you have seen before. As the amount of oxy Hb and deoxy Hb changes, the light ratio comparing red and infrared light also changes. The pulse oximeter uses this ratio to calculate the oxygen saturation.
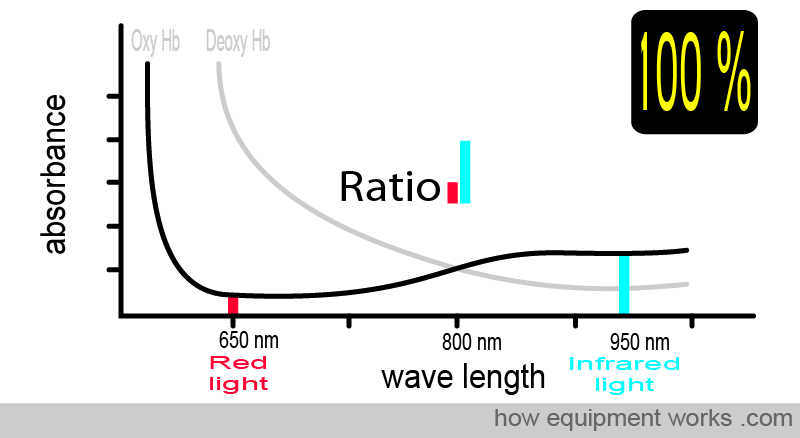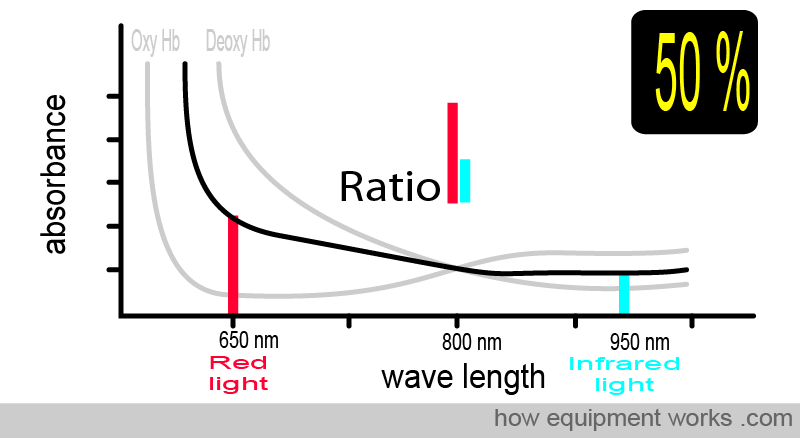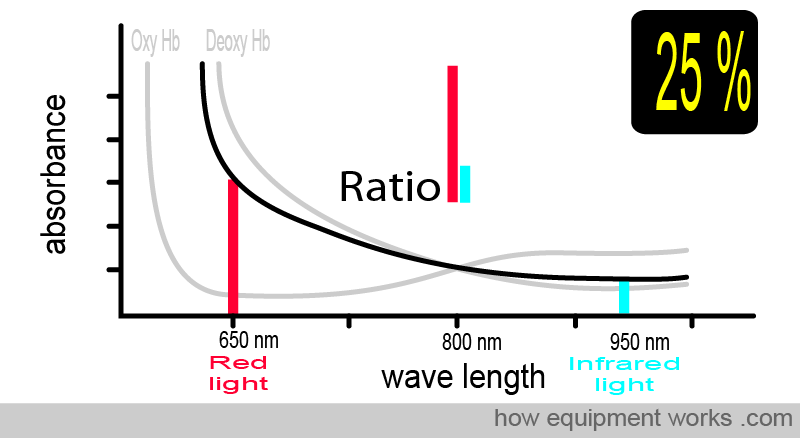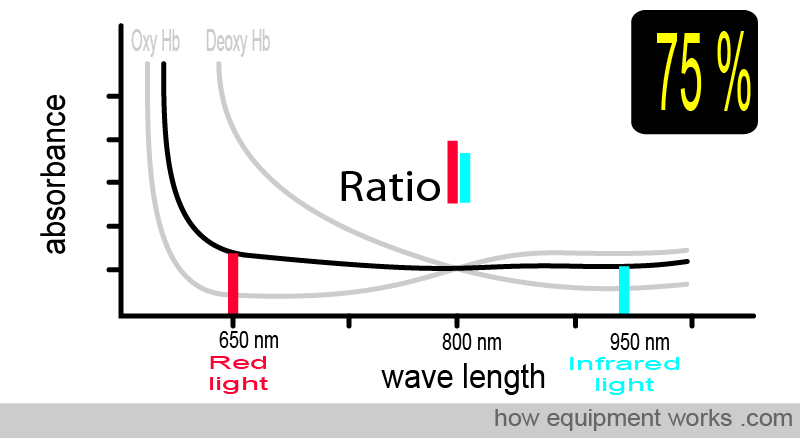
To summarise things so far, the absorbance of light depends on:
1. Concentration of the light-absorbing substance.
2. Length of the light path in the absorbing substance
3. Oxyhemoglobin and deoxyhemoglobin absorb red and infrared light differently
The pulse oximeter computer takes these factors and computes the saturation.
Calibration Adjustment
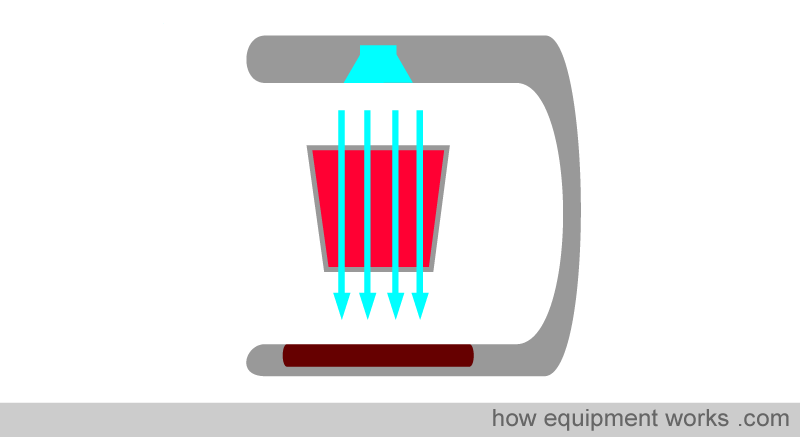
Early on, we discussed how the pulse oximeter utilises Beer’s and Lambert’s Law (absorbance depends on concentration and path length) as part of the factors it uses to compute oxygen saturation. Unfortunately, there is a problem. In physics, the Beer-Lambert law has stringent criteria for accuracy. For example, the light that passes through the sample should pass straight through, similar to the light rays in the image below.
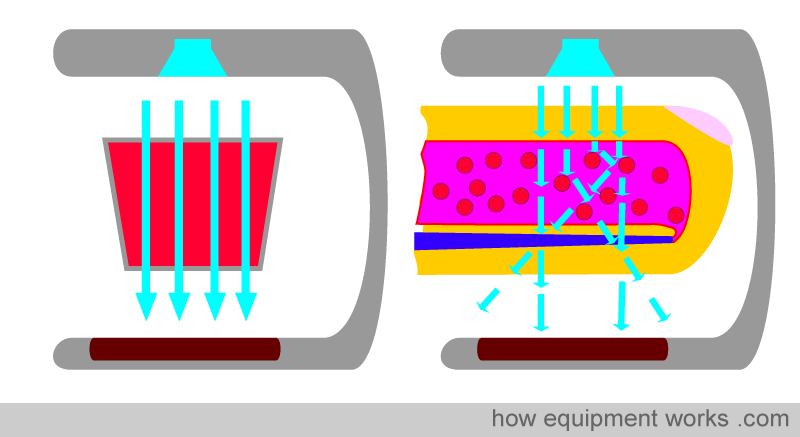
However, in real life, this does not happen. Blood is not a neat red liquid. Instead, it is full of various irregular objects, such as red cells, etc. This causes the light to scatter, rather than travel in a straight line. Therefore, Beer-Lambert’s Law cannot be applied strictly.
Because Beer-Lambert’s law cannot be applied strictly, errors would occur if it were used to directly calculate oxygen saturation. A solution to this is to use a “calibration graph” to correct for errors. A test pulse oximeter is first calibrated using human volunteers. The test pulse oximeter is attached to the volunteer, and then the volunteer is asked to breathe lower and lower oxygen concentrations. At intervals, arterial blood samples are taken. As the volunteer’s blood desaturates, direct measurements made on the arterial blood are compared simultaneously with the readings shown by the test pulse oximeter. In this way, errors due to the inability to apply Beer-Lambert’s law strictly are noted, and a calibration graph is created. However, to prevent harm to the volunteers, the oxygen saturation is not allowed to drop below approximately 75-80%.
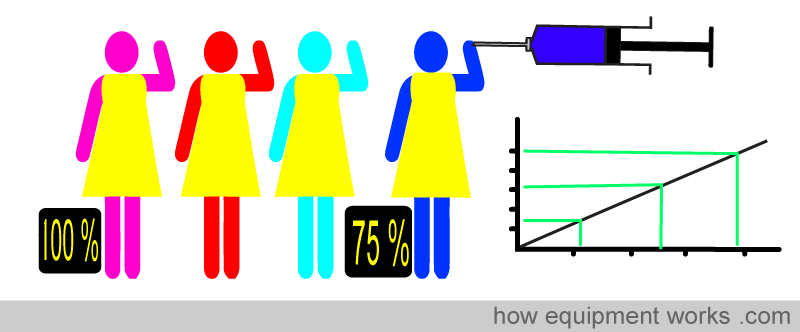
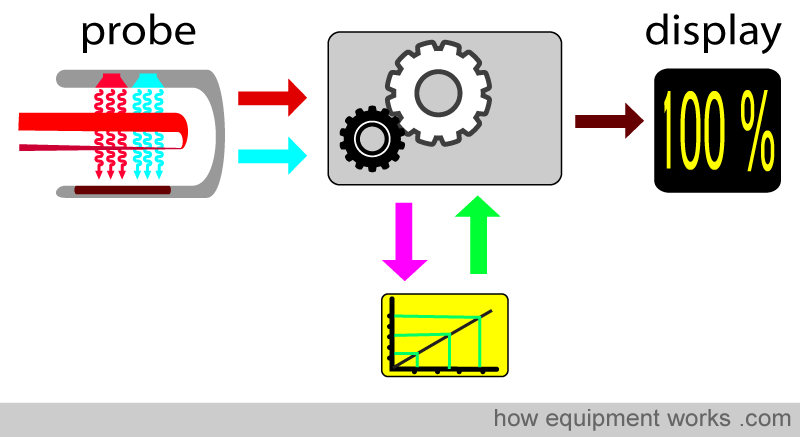
A copy of this correction calibration graph is available inside the pulse oximeters in clinical use. When doing its calculations, the computer refers to the calibration graph and corrects the final reading displayed. As mentioned before, the volunteer studies described earlier do not allow saturation to drop below about 75-80%. For saturations below this, the calibration curve is mathematically estimated. Therefore, pulse oximeters are typically less accurate below saturations of approximately 75-80%.
Please click the “Next” button below to read part 2 about how pulse oximeters work. Thank you.