Introduction
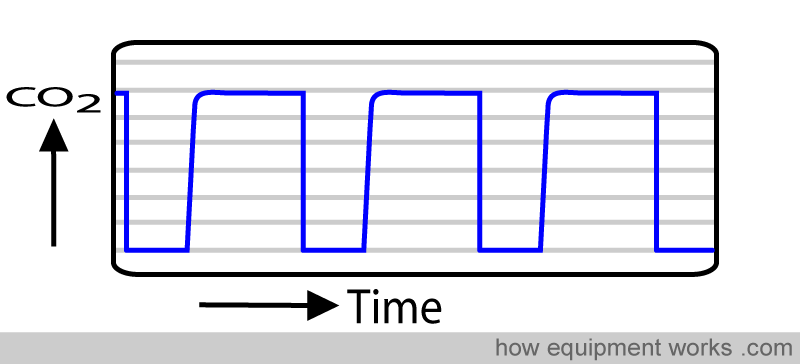
A capnograph measures how much carbon dioxide is present in the patient’s breath.
They are an essential piece of monitoring and you can find them in areas such as operating rooms, recovery, critical care, wards, and ambulances.
Advantages of capnography:
- Helps assess a variety of problems, from the cell to the breathing equipment
- Non-invasive
- Rapid
- Provide continuous measurement
- Physically small
Carbon Dioxide
We all know that oxygen is necessary for life. Oxygen goes through a series of steps before it reaches the cell
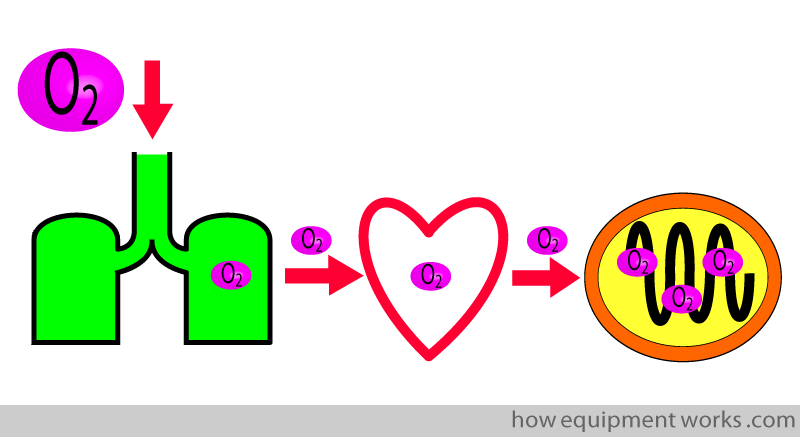
The cells use the oxygen and produce carbon dioxide (CO2) as a waste product. The CO2, like oxygen, goes through a series of steps before it is expelled out of the body.
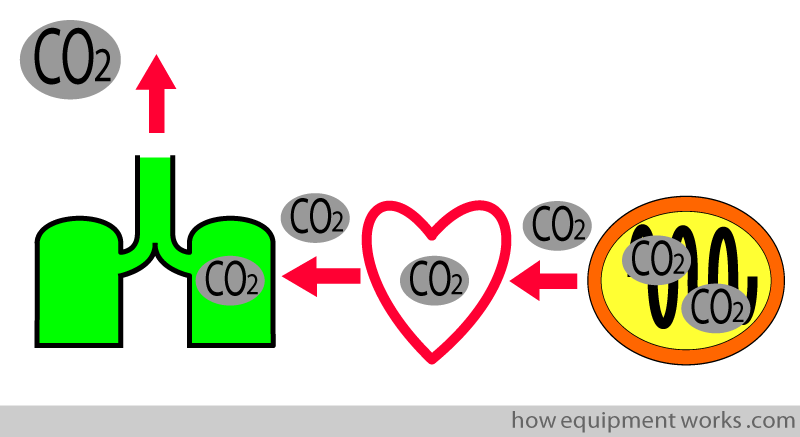
The capnograph is able to measure the expelled CO2. This is an extremely useful measurement as it can help detect problems along the pathway taken by the CO2. For example, if the patient stops breathing (e.g. due to morphine), CO2 will not be able to “get out “. This problem will make the capnograph show a low CO2 reading and trigger an alarm that alerts medical staff to the problem.
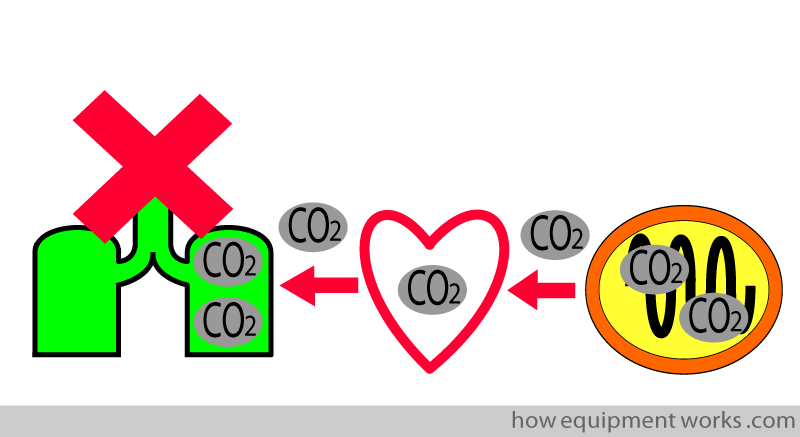
We will discuss more examples later.
Use of infrared waves
Capnography uses infrared waves to measure CO2. Infrared waves are waves that are invisible to the eye and have a lower frequency than visible light. The frequency is below red light, which is why it is called “infra” red.

Infrared is absorbed by gases that have “two or more different atoms. Oxygen gas has two atoms which are not different. Therefore, oxygen does not absorb infrared waves.
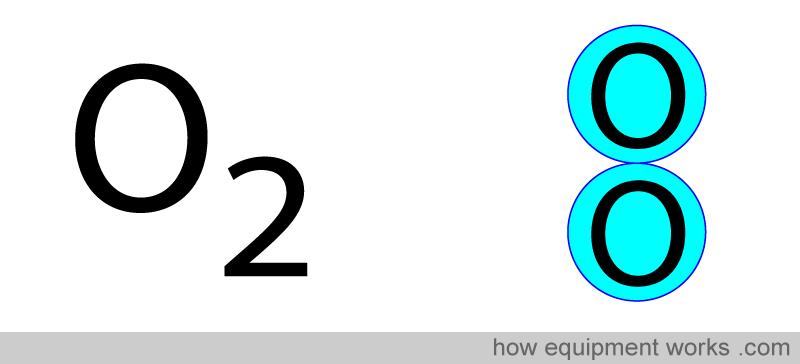
Carbon dioxide gas, unlike oxygen gas, has atoms that are different.
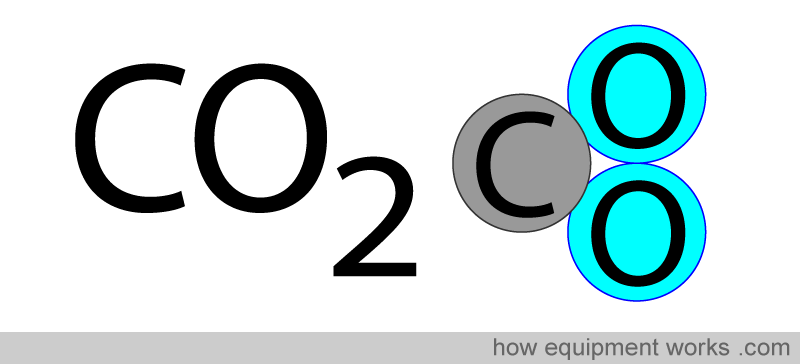
Therefore, because carbon dioxide gas has different atoms, it absorbs infrared waves.
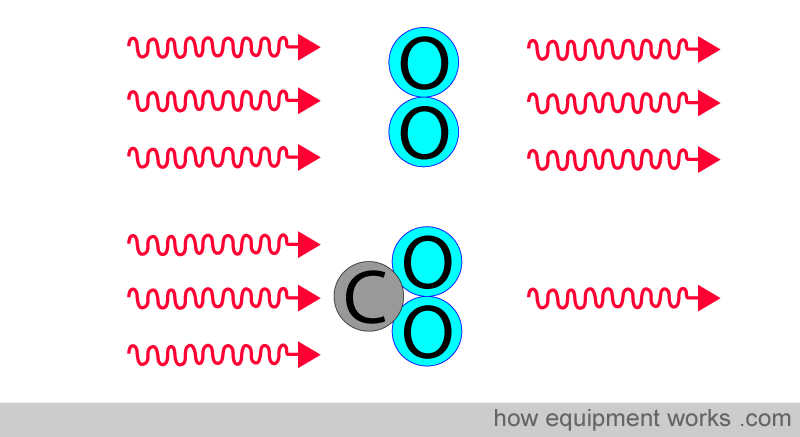
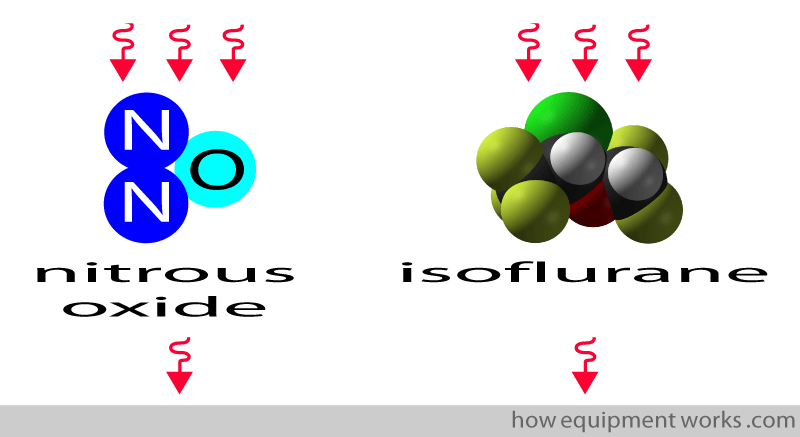
In fact, infrared absorption can be used to measure, in addition to CO2, other gases that have different atoms in their structure. For example, infrared can be used to measure nitrous oxide and isoflurane.
Basic Principle of how it works
The basic principle of the capnograph is based on infrared and the Beer-Lambert Law. This is a combination of two laws, The Beer Law and the Lambert Law.
Taking only the “Beer Law” part of the “Beer-Lambert Law” and applying it to infrared waves, it says:
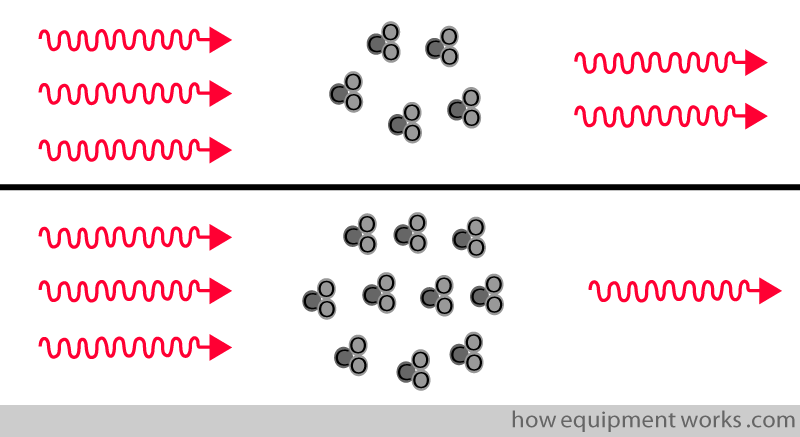
The amount of infrared rays absorbed is proportional to the concentration of the infrared-absorbing substance.
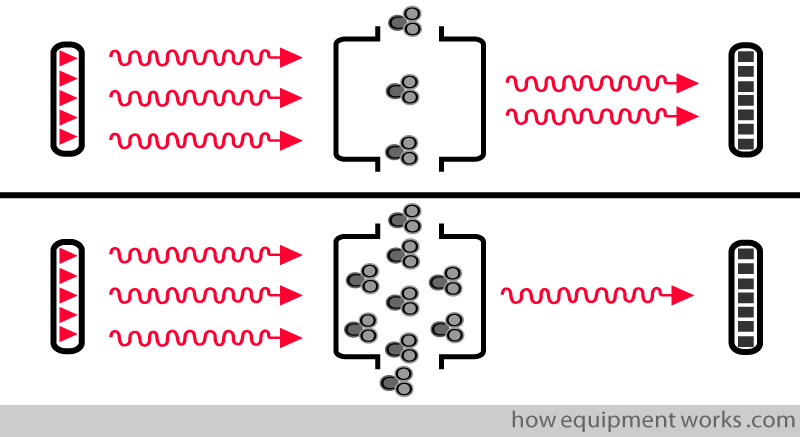
The CO2 analyser works on this principle. More the CO2 is present, the more infrared rays are absorbed.
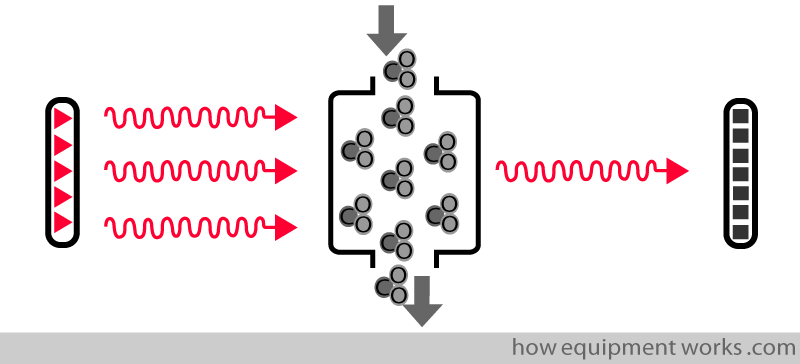
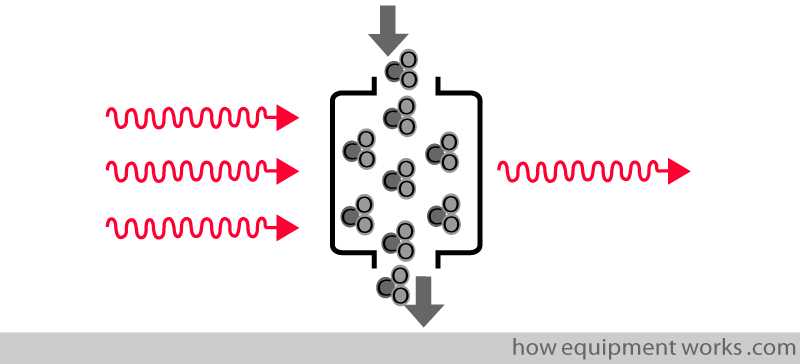
The basic analyser system consists of an infrared source, sample chamber and detector.
More the CO2 is present, the more infrared waves are absorbed.
The system will be discussed in more detail now.
Infrared source and absorption of infrared waves
The infrared source emits infrared waves.
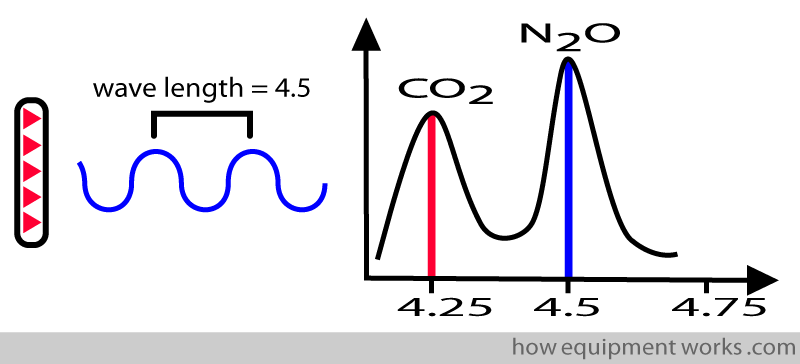
Like all waves, infrared waves have wavelengths.
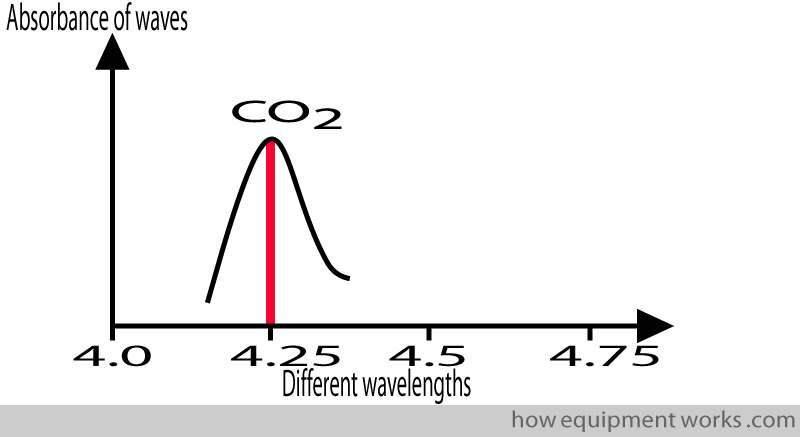
A given gas absorbs infrared waves of different wavelengths differently. If you take CO2, it maximally absorbs infrared waves with wavelengths of about 4.25 micrometres.
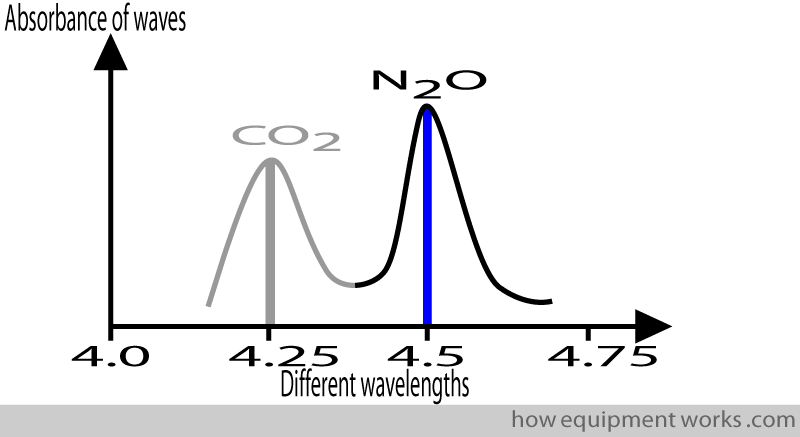
On the other hand, nitrous oxide maximally absorbs infrared waves with wavelengths of about 4.5 micrometres.
This property is useful for the system to measure a chosen gas. Therefore, to measure CO2, you will design your infrared source to emit a wavelength of 4.25. In this way, it will not measure nitrous oxide.
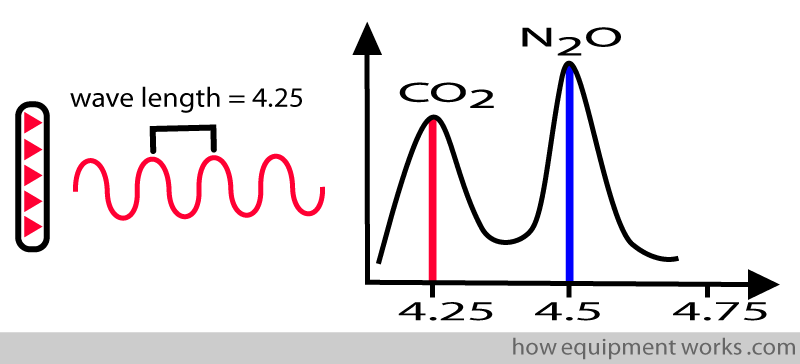
On the other hand, if you want to measure nitrous oxide gas, you will choose a wavelength of 4.5.
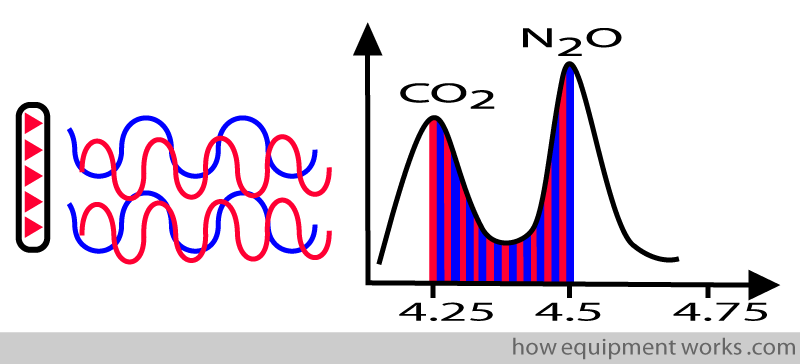
To be specific for a particular gas, the infrared source must emit waves having wavelengths within narrow limits. If the waves emitted have too much of a variety of wavelengths, the system will not be able to differentiate between gases.
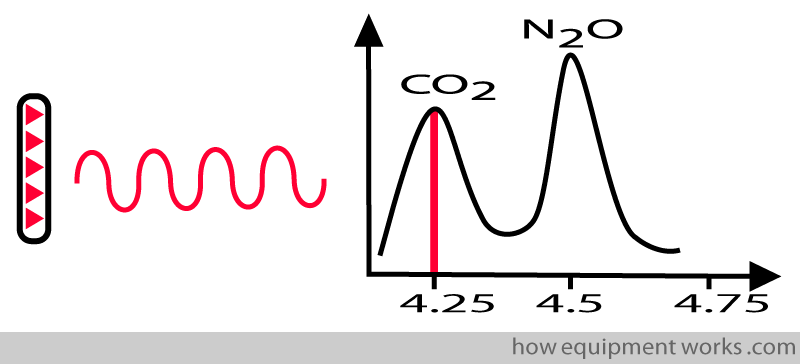
Therefore the infrared source is designed to emit waves having wavelengths within narrow limits. In more technical terms, we say that the infrared source emits wavelengths within a “narrow band “.

I am the author of this website. Let me tell you about another website I run that you may like. I am very interested in the search for happiness, and I have created a website that explains simple ideas one can use to achieve it. The website is completely free, and you are welcome to visit it at the link below.

Sample chamber
The CO2 being measured goes via a sampling chamber that has a fixed size. Not all materials allow infrared waves to pass through. Therefore the chamber is made of special material that freely allows infrared waves to pass through (e.g. sapphire).
Detector
The detector outputs a signal that is proportional to the amount of infrared waves falling on it.
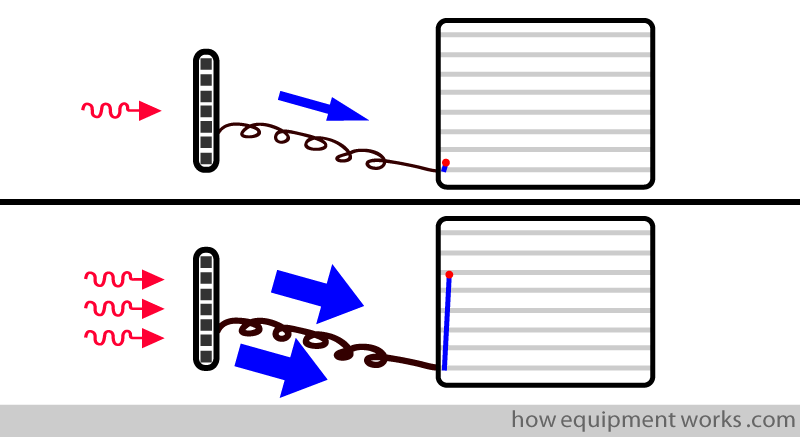
More CO2 in the sample chamber causes more infrared to be absorbed and less reaches the detector.
Less CO2 in the sample chamber causes less infrared to be absorbed and more reaches the detector.
Low CO2 = High detector infrared.
High CO2 = Low detector infrared.
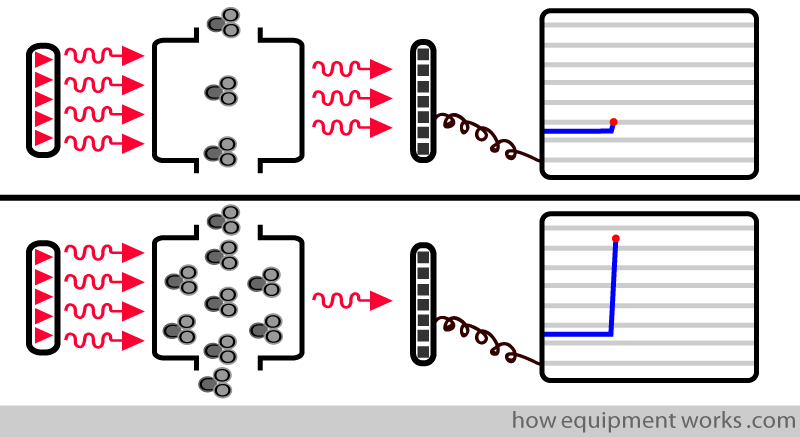
The computer uses the information from the detector to display the CO2.
Low CO2 = more infrared reaching detector = low CO2 display
High CO2 = less infrared reaching detector = high CO2 display
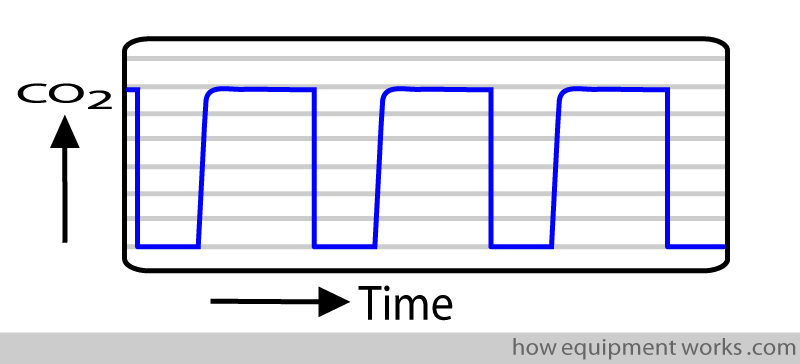
The CO2 in the sample chamber during inspiration and expiration are different. This difference over time results in the typical capnograph trace, which will be explained in more detail later.
Collision Broadening
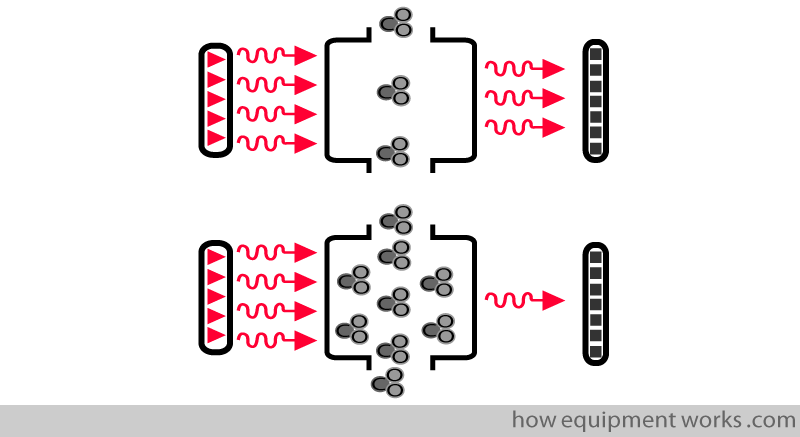
When talking about capnography, one often hears the mysterious term called “collision broadening “. I will try and briefly introduce this term to you. It was mentioned before that a gas absorbs infrared waves maximally at a certain wavelength. If we have just pure CO2 molecules (i.e. no other gases present) and subject them to infrared waves of different wavelengths, we get a narrow range of wavelengths where the infrared is maximally absorbed.
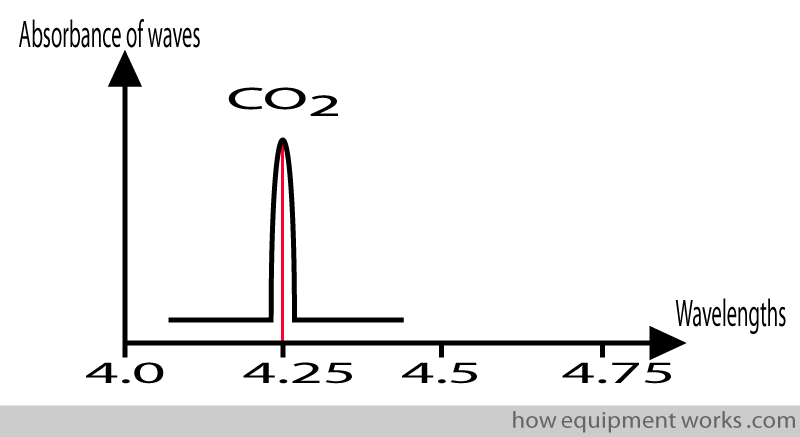
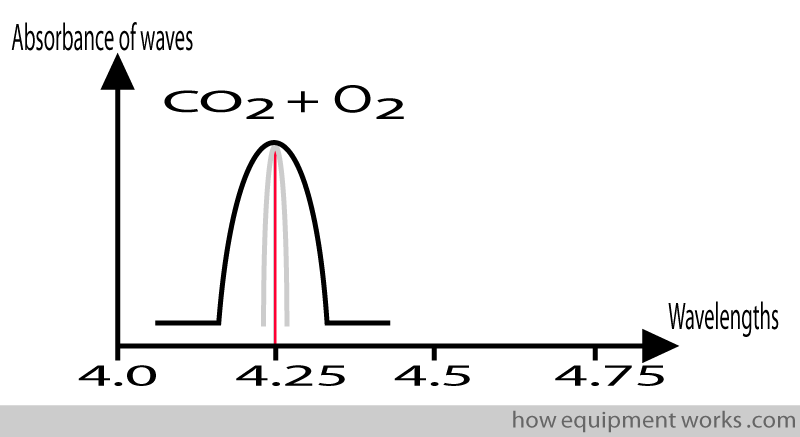
If we repeat the measurement, this time having a mixture of CO2 and another gas, such as oxygen, we will find that the absorption pattern is not as narrow as when only CO2 is present. In other words, the absorption pattern has been “broadened”.
The same happens in the presence of nitrous oxide.
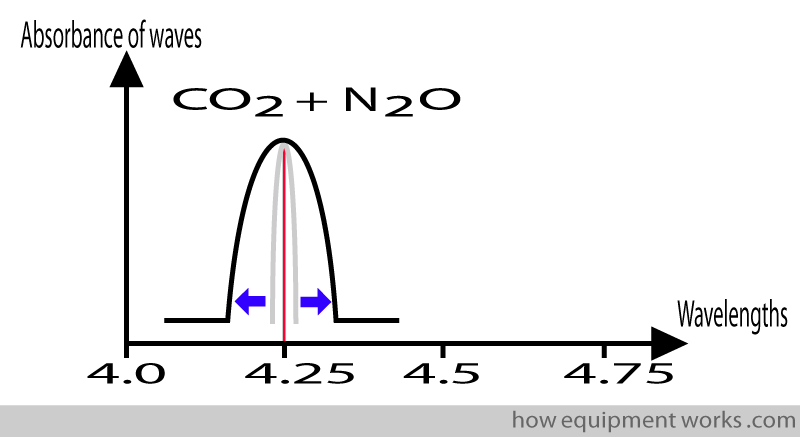
This “broadening ” of the absorption pattern is due to a variety of complicated “physics” reasons. One such reason is that the oxygen molecules “collide ” with the CO2 molecules. This results in the CO2 molecules slightly altering the way they absorb infrared waves, leading to the “broader “ absorption pattern. Because this “broadening “ of the absorption pattern occurs due to “collisions “between the gases, it is called “collision broadening “.
The relevance of collision broadening is that gases such as oxygen and nitrous oxide can affect the amount of infrared rays absorbed by CO2 leading to a potential source of error in measurement. Modern analysers measure the amount of nitrous oxide and oxygen present and use this information to correct errors due to collision broadening.
Main stream versus Side Stream
Capnograph analysers are either mainstream or sidestream.
In “mainstream” analysers, the analyser is directly near the CO2 expired by the patient. The mainstream analyser is “attached” to the patient. The analyser is connected to the monitor by long electrical wires (shown in red).
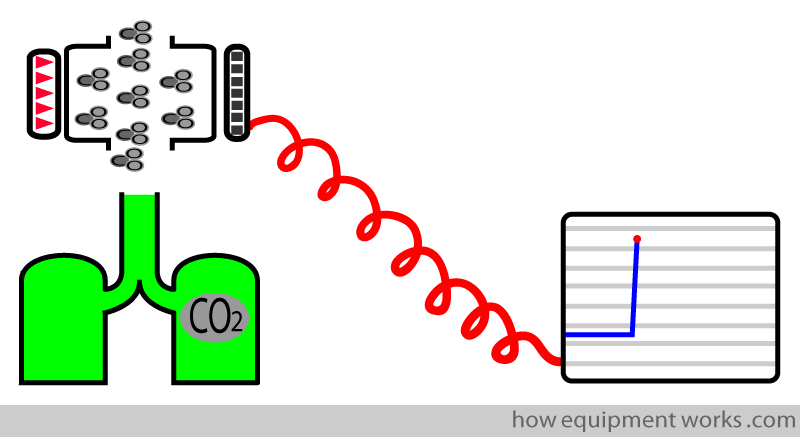
In “side stream” analysers, the analyser is away from the CO2 expired from the patient.
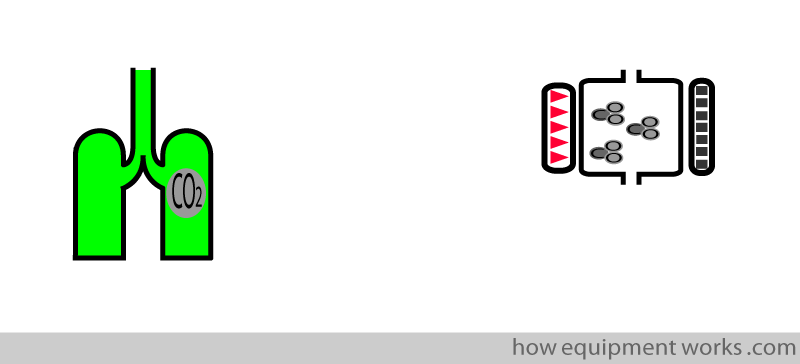
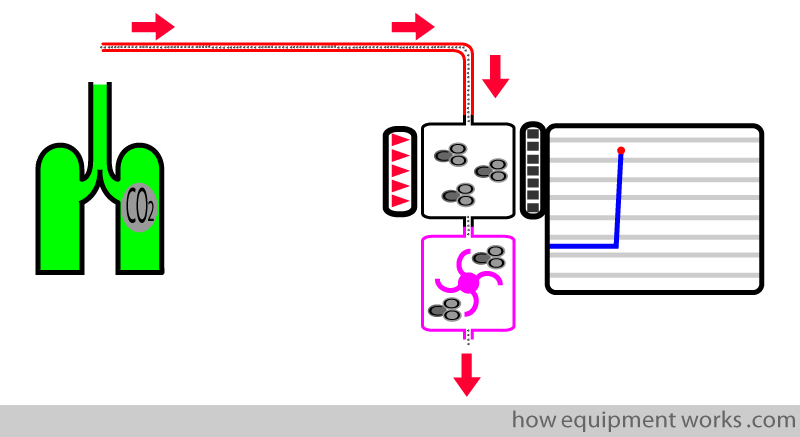
A long narrow tube (red tube shown below) is connected to the patient end. A pump (shown in pink) keeps suctioning a small quantity (e.g. 150 mL per minute) of the patient’s respiratory gases. This sample of gases flows across the analyser, which is located away from the patient.
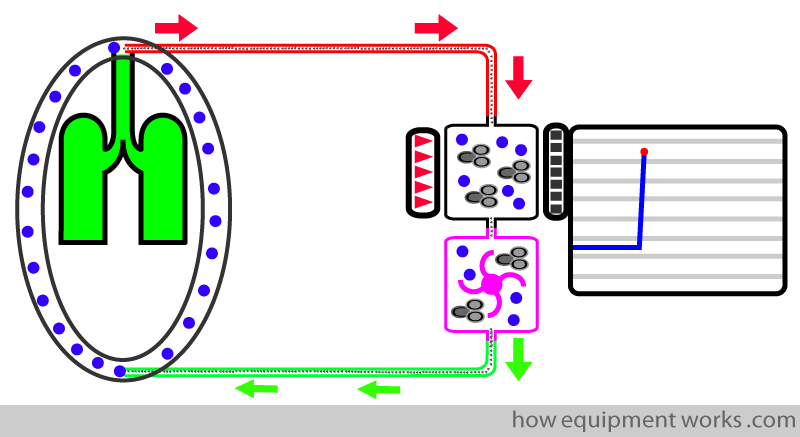
Unfortunately, the suction tubing doesn’t only suck CO2. It also sucks in expensive anaesthetic gases. To avoid wastage of the expensive anaesthetic agents, the sampled gas is returned (green arrows) to the patient’s anaesthetic breathing system.
There are advantages and disadvantages of both, mainstream and sidestream analysers. However, before discussing the differences, we need to understand “response time”
Response Time
In an ideal world, our measuring instrument should instantly show the CO2 measurement. Shown below is a response time graph. The red arrow shows the moment a test sample of CO2 is given to the measurement system. In this “ideal “ situation, as soon as the system sees the CO2, the graph rises instantly to show the CO2 measurement.
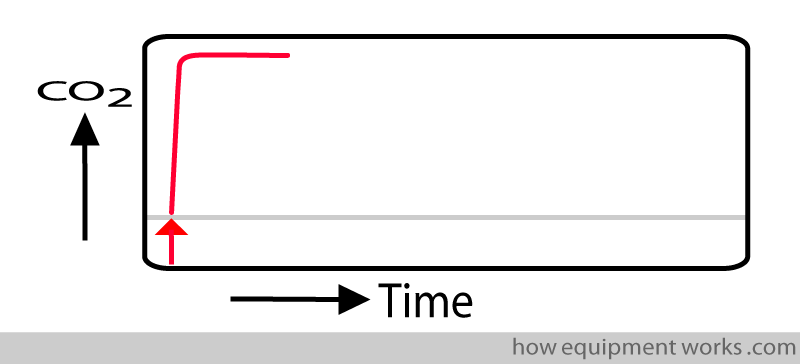
In real life, the measurement system takes time to respond to a signal such as CO2. In the response time graph below, the delayed response is shown in blue.
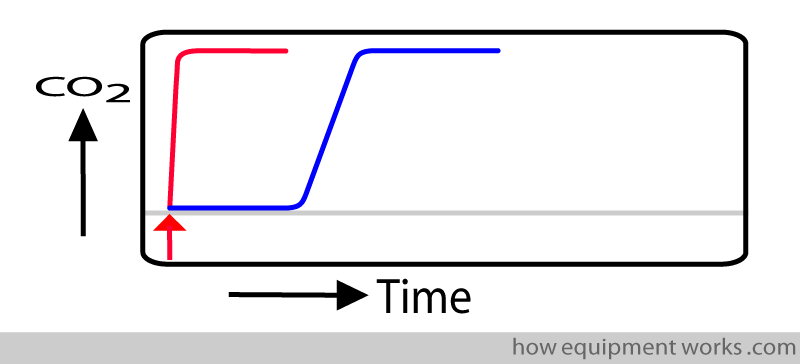
The delay in showing the measurement (response time) is due to two separate delays: Transit Time and Rise Time.
i.e. response time = transit time + rise time
Transit time:
This is the time taken by the CO2 to travel (“transit”) from the sampling end ( pink arrow) to the entrance of the analyser (blue arrow).

The transit time is represented by the straight line portion (shown in pink) of the response time graph.
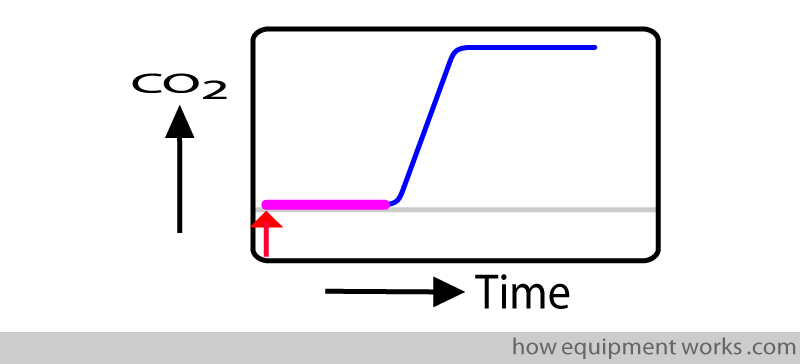
During this “transit time” the response time graph remains flat since no CO2 has yet reached the analyser.
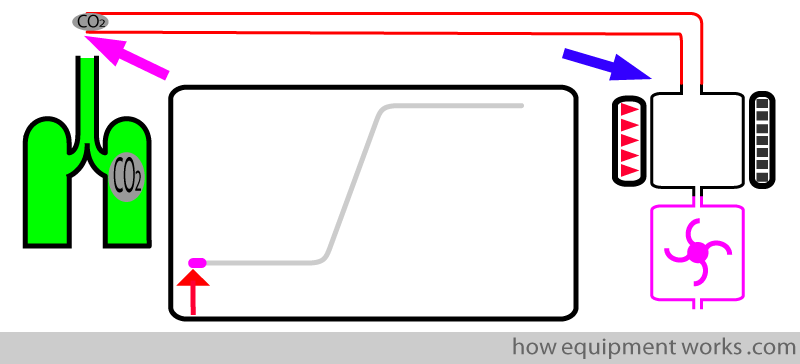
As the test CO2 travels in the tube, the trace remains on the baseline.
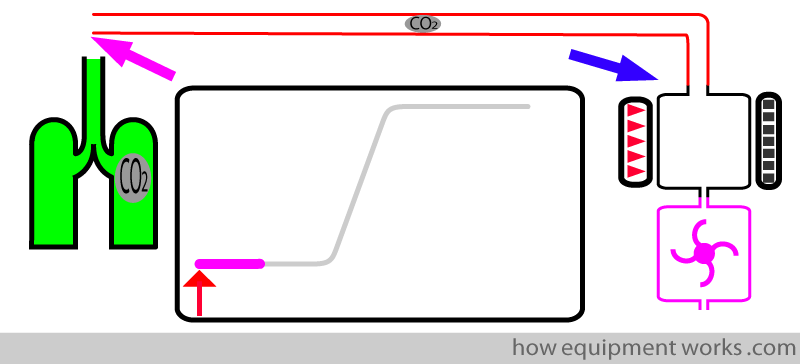
As soon as the test CO2 sample enters the sample chamber, the CO2 begins to rise (blue line). This marks the end of rise time (upright blue arrow).
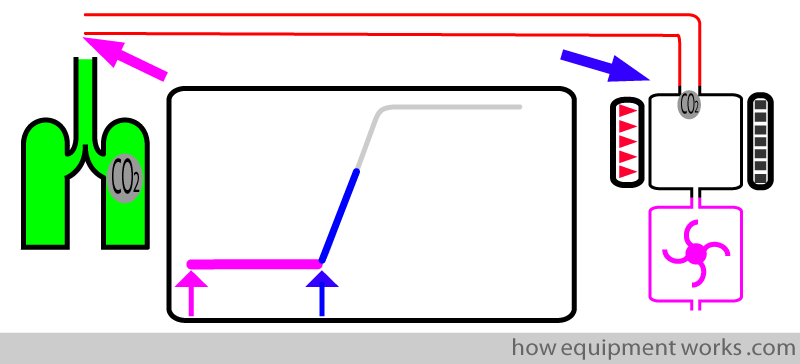
The transit time can be shortened by:
- Using a short tube.
- Using a mainstream analyser ( it has no tube, so has no transit time).
- Using a narrow sampling tube
- Using a high suction flow rate in the sampling tube (so that the gas travels faster in the tube).
Rise Time
Just to recap, response time is the delay caused by transit time and rise time.
In the last section, we discussed transit time and now will discuss rise time.
Rise time tells you how “quickly” the analyser can respond when CO2 has entered the analyser. (Remember that in transit time, the CO2 has not reached the analyser).
In the response time graph, rise time is the time required for the displayed value to rise from the baseline (minimal value) to the maximum value.
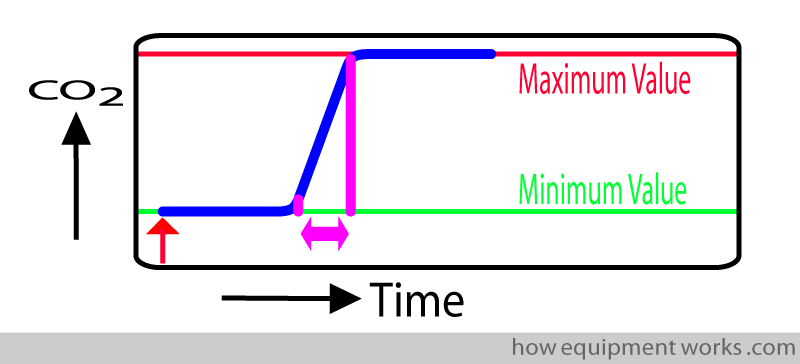
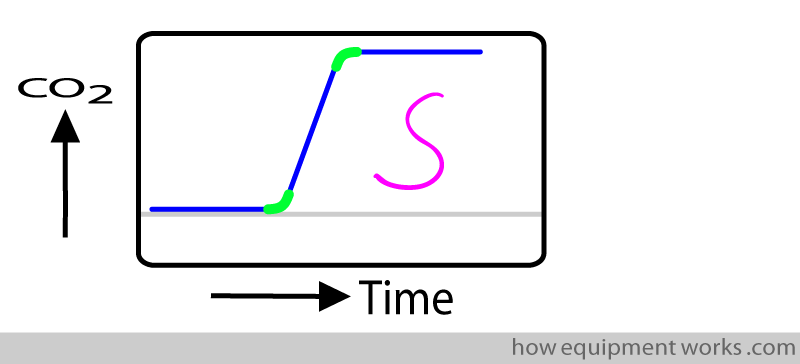
However, rise time measurement is slightly technically complicated. The problem is that the “rising” part is not actually a straight line. Rather, it is a “S” (sigmoid) shaped curve. The curved portions (green) make accurate measurement difficult.
To make it easier for the test laboratory to measure rise time, they measure rise time from 10 % of the final value to 90 % of the final value. This conveniently gets rid of the curved portions that are difficult to measure.
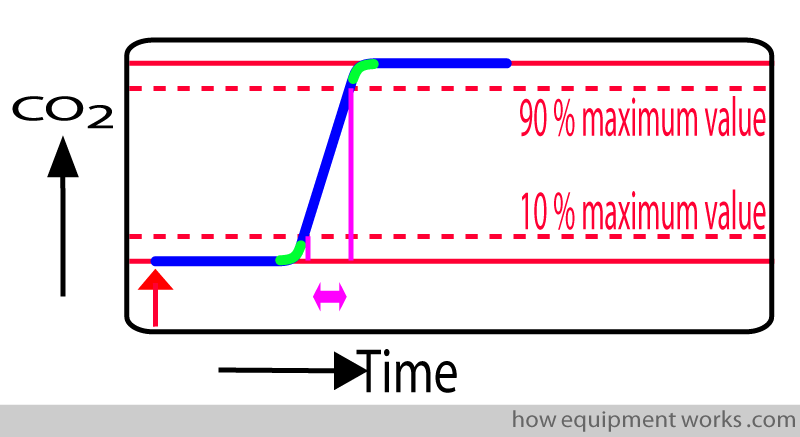
When the test laboratory mentions the rise time, they will mention the rise time as for example:
Rise time ( 10 % to 90 % of maximum) = 0.2 seconds
Rise time can be made quicker by using a smaller measuring chamber.
Please click the “Next” button below to read part 2 about how capnograph monitors work. Thank you.